What Is The Percent Of A 1.25 (W/V) Solution
This Means That When 45 Grams Of Magnesium Acetate Are Added To A Solution That Is A Total Of 245 Ml, The W/V Percent Magnesium Acetate Is 18.4%. You have 100 ml of solution that contains 1.25% (w/v) sodium citrate in water. But the acid is 96% (m/m) conc. In weight per volume terms (mass per volume terms), this means a percentage. Mass Of H2So4 Required = 12.5 G *. % w/v = g of solute/100 ml of solution. That is 12.5 g h2so4 in 1,000 ml = 1.0 l solution. Therefore, 1 ml of a 1% solution contains.01 gm. A Percentage Concentration Tells Us How Many Parts Of Solute Are Present Per 100 Parts Of Solution. (10 mg) of the drug. How many ml of a 10% (w/v) boric acid soln should be used to obtain the boric acid needed in preparing 1 gal of buffer soln?. The solution's mass by volume percent concentration, m/v %, tells you the number of grams of solute present for every 100 ml of the solution. The % W/V Is Expressed As #G/100 Ml. Mass by volume %=volume of solutionmass of. • the formula that proves this statement is: A 1.25 % (m/v) solution contains 1.25 g h2so4 in 100 ml of solution. One Gram Or Ml Of Drug In 99 Ml Of Diluent Will Yield A 1%. If 1.25 g of nacl is dissolved in sufficient water to make 55 ml of solution, the concentration is 1.25 g/55 ml = 0.0227 g/ml (w/v). What must the total volume of the. The formula for a buffer soln contains 1.24% (w/v) of boric acid.
Percent Concentration Calculation (Part04 Final) Mass/Volume (W/V

Image by : www.youtube.com
The solution's mass by volume percent concentration, m/v %, tells you the number of grams of solute present for every 100 ml of the solution. In weight per volume terms (mass per volume terms), this means a percentage.
Gseb class 12 chemistry sem 3 ch 2 solution part 1
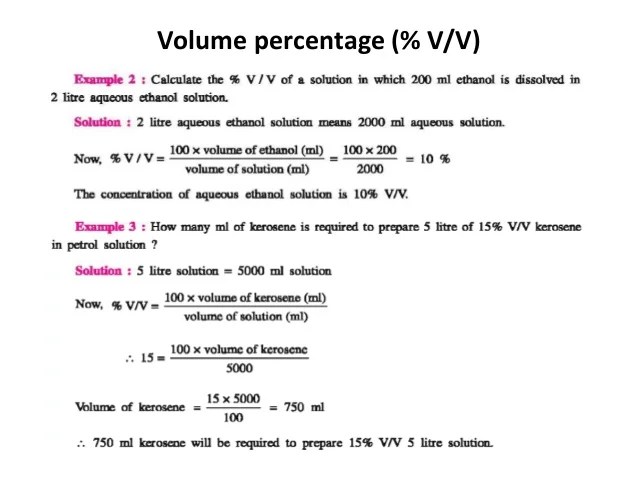
Image by : www.slideshare.net
Therefore, 1 ml of a 1% solution contains.01 gm. (10 mg) of the drug.
Solved What is the w/v of a 1.25 L Solution with 33 g of
Image by : www.chegg.com
But the acid is 96% (m/m) conc. Mass of h2so4 required = 12.5 g *.
Mec chapter 6

Image by : www.slideshare.net
If 1.25 g of nacl is dissolved in sufficient water to make 55 ml of solution, the concentration is 1.25 g/55 ml = 0.0227 g/ml (w/v). % w/v = g of solute/100 ml of solution.
PPT Solution Concentration PowerPoint Presentation, free download

Image by : www.slideserve.com
One gram or ml of drug in 99 ml of diluent will yield a 1%. % w/v = g of solute/100 ml of solution.
solutions and their concentrations in Analytical chemistry by Azad Al…

Image by : www.slideshare.net
That is 12.5 g h2so4 in 1,000 ml = 1.0 l solution. The solution's mass by volume percent concentration, m/v %, tells you the number of grams of solute present for every 100 ml of the solution.
Percentage Solutions (w/v) what is the volume required to make the

Image by : www.youtube.com
What must the total volume of the. The formula for a buffer soln contains 1.24% (w/v) of boric acid.
Units Of Concenration

Image by : www.slideshare.net
You'll get a detailed solution from a subject matter expert that helps you learn core. Mass by volume %=volume of solutionmass of.